BLX-7006.
A Best-in-Class, Oral Allosteric GLP-1 Receptor Agonist Delivering Injectable-Like Efficacy for Obesity and Type 2 Diabetes
Disease Area
BLX-7006 is being developed as an oral small-molecule therapy for patients with obesity and type 2 diabetes, addressing both excess weight and impaired glycemic control. Its goal is to deliver injectable-like efficacy in a convenient, once-daily oral pill.
Stage of Development
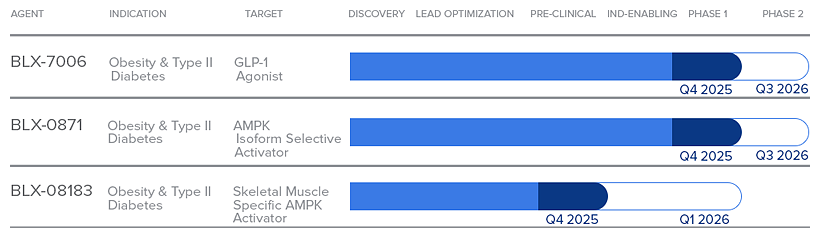
BLX-7006 is currently in Phase 1 clinical development, where ongoing studies are evaluating safety, tolerability, and pharmacokinetics in healthy adults.
Mechanism of Action
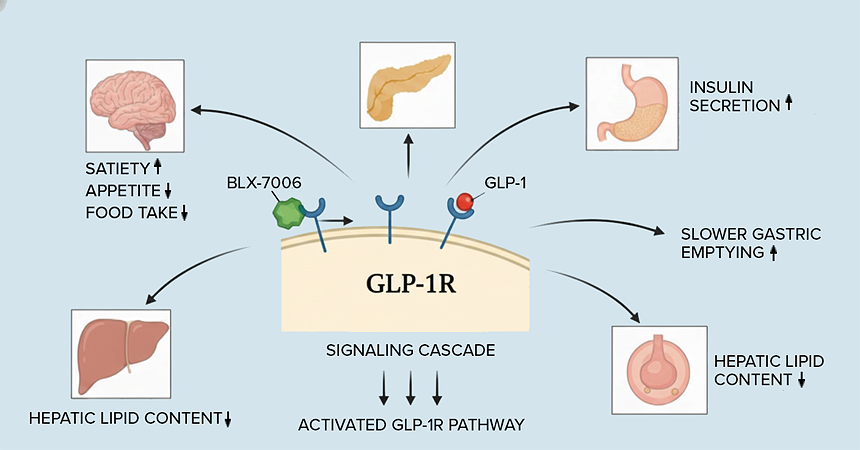
BLX-7006 is a novel, oral GLP-1 receptor agonist that mimics and amplifies endogenous GLP-1 signaling to enhance satiety, slow gastric emptying, and improve insulin secretion. It engages an allosteric binding site on the GLP-1 receptor, enabling cooperative activation with native hormone and potentially reducing desensitization seen with peptide agonists.
Differentiation
BLX-7006’s novel allosteric binding mode and distinct chemotype set it apart from later-stage oral small-molecule GLP-1 agonists, which are largely modified analogs of danuglipron or orforglipron, enabling cooperative activation of the receptor with potential for differentiated efficacy and tolerability. By targeting an alternate site on GLP-1R, BLX-7006 aims to maintain robust metabolic benefits while potentially mitigating some of the gastrointestinal limitations and dose-escalation burdens seen with current injectable and oral GLP-1 therapies.
BLX-0871.
A First-in-Class, Isoform Selective (α2/β1/γ3) AMPK Activator in Phase 1 Clinical Evaluation for Metabolic and Muscle Health
Disease Area
BLX-0871 targets major metabolic and muscle-health indications, including:
- Obesity and overweight with cardiometabolic risk, where weight loss often comes with undesirable lean mass loss
- Type 2 diabetes and insulin-resistant states, via AMPK-driven increases in skeletal muscle glucose uptake and fatty acid oxidation
- Muscle loss and reduced endurance in aging and metabolic disease, through skeletal-muscle biased AMPK activation that supports mitochondrial function, oxidative metabolism, and resistance to atrophy signals
Stage of Development
BLX-0871 is currently in Phase 1 clinical development, where ongoing studies are evaluating safety, tolerability, and pharmacokinetics in healthy adults.
Mechanism of Action
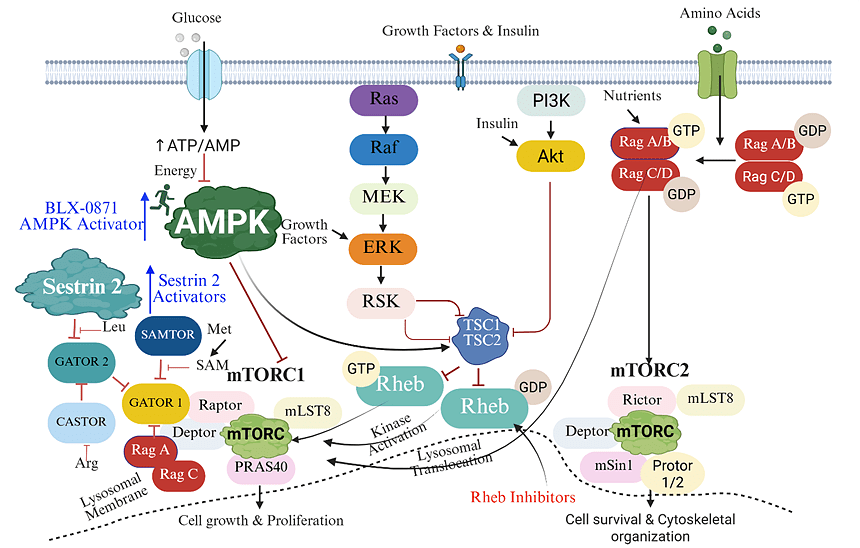
BLX-0871 is an oral small-molecule AMPK activator designed to selectively target the γ3- containing AMPK complexes in skeletal muscle, the key heterotrimers that drive muscle glucose uptake. By preferentially binding the ADaM site of γ3-rich complexes, it enhances fatty-acid oxidation, stimulates GLUT4-mediated glucose transport, and boosts mitochondrial function and endurance capacity, acting as an exercise mimetic. This muscle-targeted, isoform-selective profile avoids AMPK activation in the liver and heart.
Differentiation
BLX-0871 is differentiated from earlier AMPK activators and existing obesity/diabetes therapies through its unique isoform selectivity that avoids cardiac side effects seen with pan-AMPK activators, its muscle-sparing profile that preserves lean mass during weight loss, and its exercise- mimetic properties that enhance metabolic function.
BLX-08183.
An Isoform-Selective (α2/β2/γ3) AMPK Activator in IND-Stage Development for Metabolic and Muscle Health
Disease Area
BLX-08183 targets skeletal muscle metabolic dysfunction associated with aging, sarcopenia, and related metabolic decline. It aims to restore muscle energy homeostasis, endurance, and mitochondrial efficiency without affecting cardiac tissue. The molecule is positioned for skeletal muscle-focused metabolic enhancement applications.
Stage of Development
BLX-08183 is currently in advanced preclinical development with demonstrated in vivo target engagement and muscle-specific AMPK activation biomarkers. IND-enabling studies are in progress to support entry into Phase 1 trials.
Mechanism of Action
BLX-08183 is a selective activator of the AMPK α2/β2/γ3 heterotrimer complex, a dominant isoform in skeletal muscle. Through direct allosteric binding and modulation, it enhances mitochondrial biogenesis, fatty acid oxidation, and glucose uptake in muscle fibers.
Differentiation
Unlike pan-AMPK activators such as MK-8722, β1 activator PF-06409577 or γ3 activator PF- 07293893 compounds that trigger cardiac hypertrophy and broad systemic activation, BLX-08183 exhibits strict skeletal muscle selectivity and an excellent cardiac safety profile. Its novel chemical scaffold delivers potent energy restoration without off-target liabilities.
Muscle-Sparing Weight Loss:
Combination Trial.
Muscle-Sparing Weight Loss: Phase 1 Combination Trial of BLX-7006 and BLX-0871 in Obesity and Type 2 Diabetes
Overview
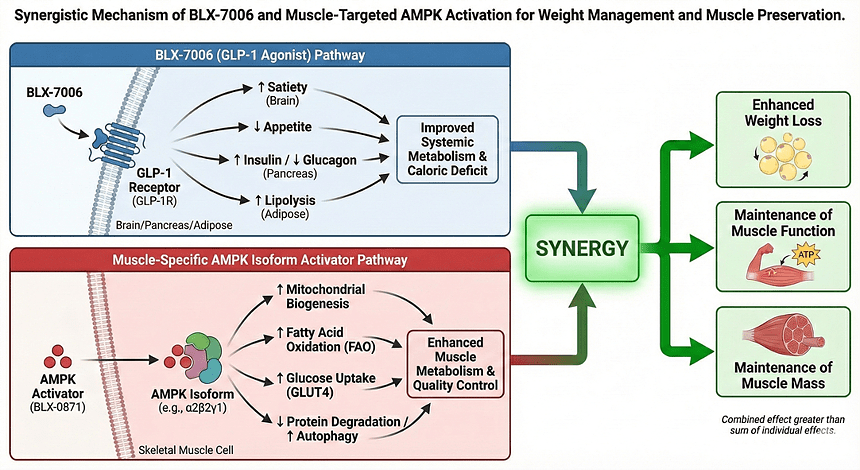
Biolexis is advancing a differentiated, all-oral dual-mechanism program that combines BLX-7006, a novel allosteric GLP-1 receptor agonist, with BLX-0871, a first-in-class, isoform-selective AMPK activator, with the goal of delivering high-quality weight loss and muscle preservation in patients with obesity and type 2 diabetes who are at risk of muscle loss.
BLX-7006 Single-Agent Summary
BLX-7006 is a once-daily, oral small-molecule allosteric GLP-1 receptor agonist designed to enhance satiety, improve glycemic control, and enable convenient, needle-free therapy for obesity and type 2 diabetes. Preclinical studies demonstrated robust weight loss in diet-induced obese mice and a favorable GLP toxicology profile in rats and non-human primates, supporting a broad therapeutic window and progression into Phase 1 single-ascending and multiple-dose trials in healthy adults.
BLX-0871 Single-Agent Summary
BLX-0871 selectively activates α2-containing AMPK complexes predominantly expressed in skeletal muscle and adipose tissue, aiming to increase energy expenditure, preserve lean mass, and avoid the cardiac isoforms that limited earlier pan-AMPK activators. In preclinical models, BLX-0871 produced meaningful weight reduction while maintaining a high proportion of lean body mass.
Path to Combination Phase 1
The strategic objective is to transition from separate first-in-human programs to a rational, data- driven combination Phase 1 trial once monotherapy Phase 1 is complete. The planned combination Phase 1 study in 2026 will enroll adults with obesity, with or without type 2 diabetes, at risk of or experiencing muscle loss, and will evaluate safety, tolerability, PK, and exploratory efficacy endpoints to confirm that the BLX-7006/BLX-0871 regimen can achieve substantial weight loss with preservation of lean mass in the target patient population.